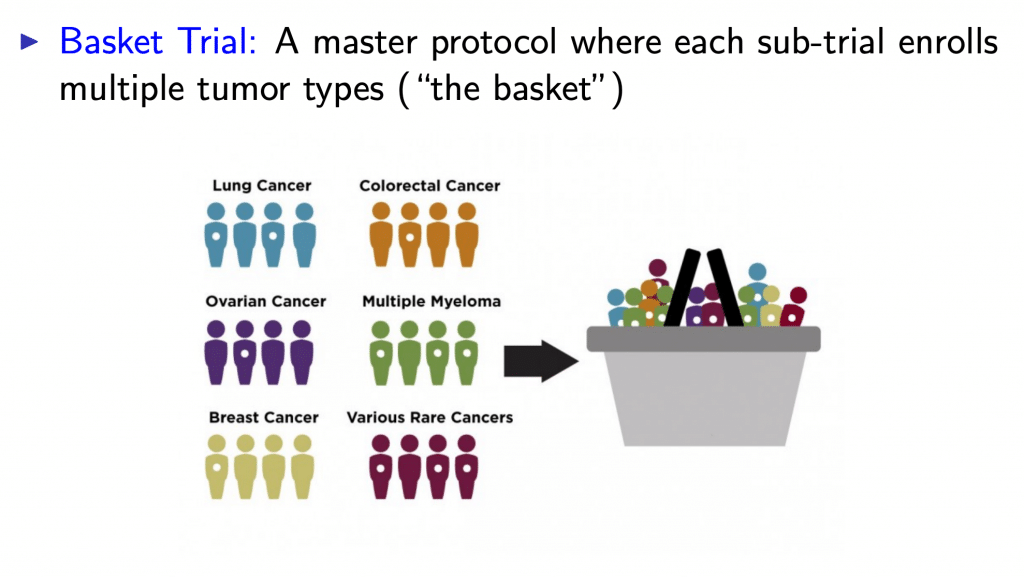
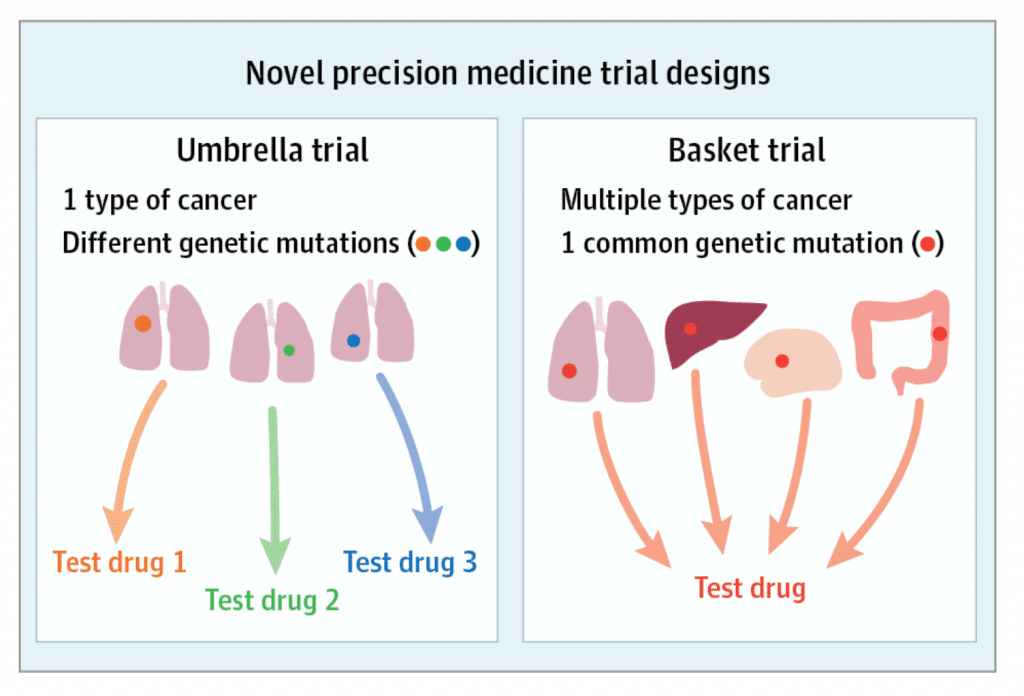
Basket Trials for Cancer Treatments
By Brent Wilson, PhD
Types of Trials
A clinical trial can take many structures. A single-arm trial is perhaps the simplest, which measures a response to therapy over time. This may have several shortcomings, such as how the group would respond over that same time without treatment (or with a placebo). Single-arm trials are most often used in the field of oncology. This is because it would be unethical to provide only a placebo for the vast majority of cancer patients.¹
Recent developments in oncology and especially immunotherapy, have helped make progress in treating previously intractable cancers.² However, these treatments have often been incredibly useful to some patients and had little effect, or even harmful effects, for other patients. As a result of this incredible variance in response, genomics has been increasingly deployed in order to better predict which patients will benefit from a given treatment.
Basket Trials
A basket trial is conducted on the hope that the previous segmentation of cancers based on cancer-type, or cancer tissue, provides an incomplete picture of how to treat patients. It may be more useful to group patients based upon genetic information: often a particular mutation.
In contrast, an umbrella trial bears more resemblance to the approach prior to genetic characterization. It enrolls patients based upon where their tumor is located.
One of the early successes of tissue independent profiling was published in 2014, making it a relatively recent method.⁴ A method of treatment decision using molecular profile across cancer tissues is described within this study. A multi-factorial assessment of each patient utilizing mutations, copy number variation, and IHC (estrogen, progesterone and androgen receptors) analysis showed success by their criteria of “safe, feasible, and compatible with clinical practice.” The authors, however, believed that additional, and more validated biomarkers would improve the approach.
More recently, the MyPathway study (published in 2018)⁵ showed that targeted therapies could work for off-label tissue types for significant number of patients (23%). It was based upon HER-2 (and other) mutations. The authors of the publication admit previous basket trials had shown mixed results. They also said their trial did produce “meaningful responses in patients with various tumor types.” The result of 23% of patients producing a response, in our view, certainly could be improved with a more accurate set of biomarkers.
The Need for Better Biomarkers
Biomarkers are an important factor in the success of basket trials, and can not be understated. Building a multidimensional biomarker, rather than relying on a single analyte like PDL1, provides greater predictive power and accuracy. Learn more about ImmunoPrism®, which utilizes Predictive Immune Modeling to build multidimensional biomarkers to accurately predict response to therapies.
References:
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3059315/
2. https://www.frontiersin.org/articles/10.3389/fonc.2019.00229/full
3. https://cddf.org/files/2018/03/02-Lindsay-Renfro.pdf