The Complexity of PD-L1 Biology
By Kevin Flanagan, PhD
PD-1 and PD-L1 inhibitors have growing importance in cancer treatment. Many tumors express PD-L1, a cell surface protein that binds to PD-1. PD-1 is expressed by T cells, an immune cell type important for fighting infections and cancer. When tumor PD-L1 binds to T cell PD-1, it inhibits the T cell response. Tumors use this pathway to escape clearance by the immune system. PD-1 and PD-L1 inhibitors block this PD-1—PD-L1 interaction, enabling a strong T cell anti-tumor response. PD-1 and PD-L1 inhibitors are in a class of drugs called checkpoint inhibitors. Checkpoint inhibitors block cancer’s ability to evade the immune response, allowing a patient’s own immune system to target and hopefully destroy the cancer cells.
The Problem with PD-1 and PD-L1
Unfortunately, PD-1 and PD-L1 inhibitors are only effective for a minority of patients. To predict which patients will respond to these treatments, doctors and pharmaceutical companies have turned to biomarkers. However, many currently used biomarkers are poorly predictive of patient response. For example, up to 20% of lung cancer patients respond to these inhibitors. For patients with PD-L1-positive tumors, the response rate is only 45% according to the highest estimates. Given that ~55% of patients who are predicted to respond to treatment do not respond, the marker has poor positive predictive power. PD-L1 status also has poor negative predictive power. A meta-analysis found an overall response rate of 13% in PD-L1-negative patients. These patients are “false negatives” because the status incorrectly classifies them as non-responders.
The high number of false positives and false negatives speaks to the need for more robust biomarkers. It also alludes to the complexity of tumor biology. In particular, patients with PD-L1 tumors who respond to anti-PD-1/PD-L1 therapy raise questions about the mechanism of action of these treatments. If these drugs work by interrupting this interaction, how can a patient respond when they lack tumor PD-L1 expression? How can a drug block a pathway that isn’t present?
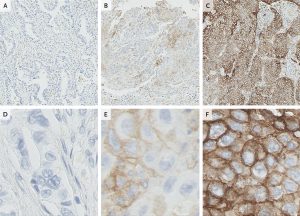
The answer is probably multi-faceted. Contributing to the disparity between PD-L1 biomarker status and inhibitor response are the discordant definitions of “PD-L1-positive”. Some studies require 50% of tumor cells to be positive. In others, 5% is sufficient. In still others, only 1% positive staining is adequate. The same patient could be positive in one study but negative in another. Further, the methods and antibodies used to measure tumor cell PD-L1 expression also vary, potentially affecting the results. As a result, tumors can be classified as PD-L1-negative even when they do express PD-L1.
Additionally, PD-L1 expression is typically measured on cancer cells only. That means that non-cancerous cells associated with the tumor can express the protein even in a tumor classified as PD-L1-negative. For instance, immune cells such as T cells, dendritic cells, and macrophages also express PD-L1 and may contribute to the therapeutic effects of PD-1/PD-L1 inhibitors, including in PD-L1-negative tumors. In fact, a recent study found that expression of PD-L1 on macrophages is correlated with overall survival.
The Role of NK cells
Natural Killer (NK) cells, an important anti-tumor immune cell type, may also play a role in anti-PD-1/PD-L1 therapeutic response. Like T cells, NK cells express PD-1. Also like T cells, PD-1-expressing NK cells can be inhibited by PD-L1-expressing tumor cells. The effectiveness of PD-1 and PD-L1 inhibitors in PD-L1-positive patients may rely in part on inhibiting this interaction. In addition, NK cells can upregulate PD-L1 in the presence of tumor cells. These PD-L1-positive NK cells can act as immune regulators by inhibiting dendritic cell maturation and subsequent priming of T cells to recognize tumors. This PD-L1-positive NK cell inhibition of the anti-tumor immune response occurs via the PD-1/PD-L1 interaction. The inhibitors might block this immune inhibition and increase the anti-tumor immune response.
In contrast, some NK cells express PD-L1 when activated. These PD-L1-positive NK cells have increased cytotoxic activity, i.e., are more potent tumor cell killers. Treatment of PD-L1-positive NK cells with the inhibitor Tecentriq (atezolizumab) increases this cytotoxicity. Treatment of PD-L1-negative tumors with Tecentriq shrinks tumors in wildtype mice, but not in mice lacking either PD-L1 or NK cells. Interestingly, in this context the PD-L1 inhibitor acts not by disrupting the interaction between PD-1 and PD-L1 but by enhancing NK cell activation via intracellular signaling. This novel mechanism of action for this inhibitor is divorced from tumor PD-L1 expression. More work is needed to understand the it’s role in NK cells, but these and other recent findings suggest important functions for NK cells in anti-PD-1/PD-L1 therapies.
Looking Forward
Poor definitions of PD-L1 status along with the it’s activity in immune cells may explain why some patients with PD-L1-negative tumors respond to PD-1/PD-L1 inhibitors. Continued work is needed to better understand the complex biology underlying the mechanisms of cancer immunotherapies. An incomplete understanding of how drugs, cancer, and the immune system interact can lead to inadequate biomarkers like PD-L1. Cofactor Genomics’ ImmunoPrism® uses Health Expression Models to develop multidimensional biomarkers with the potential for improved predictive power relative to single-analyte biomarkers like tumor PD-L1. Better biomarkers will help more patients to benefit from the promise of immunotherapies.