Hello Blog World! I am a new molecular biologist at Cofactor Genomics, and I must admit that I haven’t been around RNA Seq applications long enough to write the stellar blog posts that my colleagues produce week after week (see here, here, or here) describing the intricacies of the technology. I do, however, have a huge interest in cancer biology since my PhD work at Washington University in St. Louis (lab of Dr. Jason Weber) focused on this topic. As a result, my blog topics will likely focus on cool new research that I find in the literature, both cancer and non-cancer related.
My PhD work led to the discovery that a signaling pathway cells usually use to fight off viral infections, called the Type I interferon response, can actually drive the proliferation of certain tumor cells. My work got a little bit of media coverage if you are interested in a non-scientist version of the story. This discovery was pretty exciting because there are numerous drugs (including some that are already FDA approved for the use in other diseases) that target this signaling pathway, so the potential for someday helping patients exists.
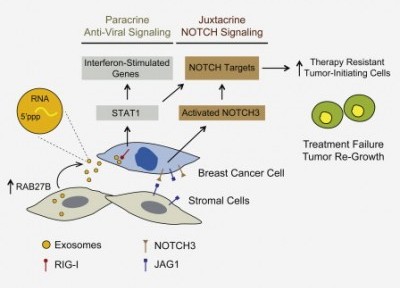
Anywho, I’m done tooting my own horn. I only mention that work because I wanted to highlight a recent paper that made some really great observations regarding exosomes, which we have been thinking a lot about here at Cofactor. In the paper, found in a recent edition of Cell, a group from the Netherlands Cancer Institute showed that exosomes secreted from tumor stromal cells (these are essentially all the supportive cells that make up a tumor that are not actually cancer cells themselves) can induce a similar type I interferon response in breast cancer cells which leads to resistance to both chemotherapy and radiation therapies.
What I find incredibly interesting about these findings is that RNA molecules packed within these exosomes are the driving force behind the induction of the interferon response through activation of the cytosolic receptor known as RIG-1. How can RNA transcribed from our own DNA elicit an interferon response (bearing in mind that typically the type I interferon response is reserved for responding to foreign intruders like viruses)? What sort of evolutionary advantage might this bestow on an organism? The answers to these questions aren’t well-defined yet, but there is already some pretty intriguing data from mice. In a PNAS paper published last year, Leonova and colleagues describe how the tumor suppressor p53 cooperates with DNA methylation to silence regions of our genome containing short, interspersed nuclear elements (SINEs). When normal p53 function is lost, which occurs in a high proportion of human cancers, these elements become de-repressed (nerd jargon meaning “turned on”) and their transcription induces the interferon response. With these observations in mind, it’s possible to imagine a situation where tumor stromal cells have de-repressed these genomic loci for some unknown reason. As a result, RNA molecules that can elicit an interferon response are present in greater ratios in the cytosol, leading to their loading into exosomes and subsequent release.
The above image was taken from: Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Boelens, Mirjam C. et al. Cell , Volume 159 , Issue 3 , 499 – 513.
So surprisingly, it appears that the end result of sensing the immune response is resistance to common cancer therapies (at least for these particular breast cancer cell types). This is certainly not what one would call an “evolutionary advantage,” unless you are a tumor cell. Perhaps there’s a benefit for such a mechanism during development that have yet to be discovered.
I think the take home message of these papers is that we need to keep an open mind about the cargo being delivered in exosomes. At this point, we really have no idea how and if loading is regulated, and variations in both spatial and temporal exosome formation could have dramatic effects on what types of RNA molecules or proteins are being secreted. Moreover, how different cell types respond to different exosome cargos is a complete mystery.
What other secrets are exosomes hiding from us? We would love to find out, so contact one of our project scientists to discuss how we can help you answer these fascinating questions.
Oh, and stay tuned, because next time I’m going to talk about mole rats. Naked ones. Why, you ask? Because they don’t get cancer, and that blows my mind. Are you on the edge of your seats now? Have a great week everyone!