Tumor Infiltrating Lymphocytes Immunotherapy
Tumor-infiltrating lymphocytes (TILs) are lymphocytes that directly infiltrate the tumor microenvironment (TME) opposing or surrounding tumor cells and in some cases subsets of cancer-reactive T-cells with killing activity clonally expand and mount an anti-tumor response. The degree of TIL infiltration is measured by both the extent and density of the TIL infiltrate using standardized systems that have been defined for objective grading of the degree of infiltration.¹ Accurate TIL measurement is critical as the extent of infiltration has been associated with favorable outcomes for a variety of tumors.2,3,4 Furthermore, the revolutionary emergence of immune checkpoint inhibition to treat cancers is limited to tumors that exhibit endogenous populations of TILs at sufficient frequencies that can be sufficiently reinvigorated to recognize tumor antigens and mediate tumor killing. For tumors where the extant population of TILs is absent or fails to function, other immune modulating therapies such as adoptive T-cell transfer, engineered T-cell therapies or cancer vaccines can be used to augment the native TIL population in the anti-tumor response. Accordingly, measurement of tumor-infiltrating lymphocytes infiltration is key to informing the most effective therapeutic options and patient outcomes.
In combination with tumor-infiltrating lymphocytes, PD-1/PD-L1 expression is also a common parameter for evaluating the tumor microenvironment (TME).5 Though the importance of PD-1/PD-L1 expression has been demonstrated in some cases (e.g., registration of pembrolizumab for non-small cell lung cancer patients with PDL1 expression in >50% of analyzed tumor cells6), its well-established variability, both temporally and spatially, limits its applicability as a predictor of response. Indeed, it is likely that PD-1/PD-L1 expression is just one of many multivariates in the TME that mediate response and it has been suggested that more relevant correlates might include the overall mutational burden of the tumor (TMB) in addition to the clonal diversity of the tumor-infiltrating lymphocytes population and to the expression of PD-1/PD-L1 on the infiltrating lymphocytes themselves.7,8 Moreover, other important attributes of the TME, such as the presence of tumor-associated macrophages (TAMs) or myeloid-derived suppressor cells (MDSCs), are currently underestimated and not included in most of the currently used classifications of immune infiltrates.9
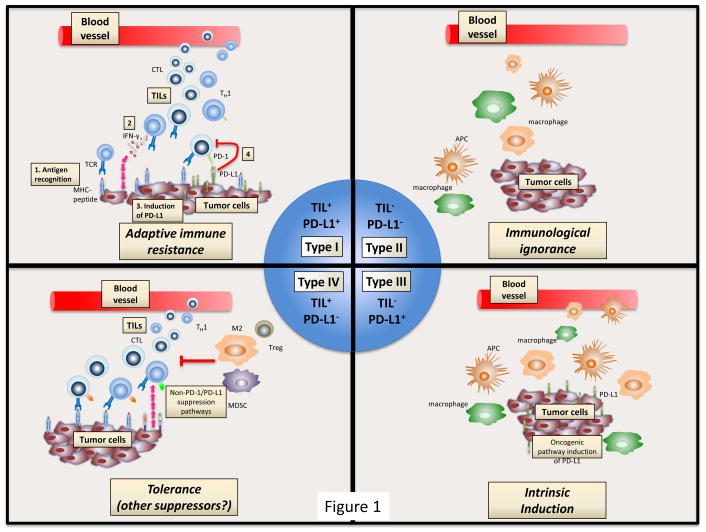
Source: https://www.ncbi.nlm.nih.gov/pubmed/25977340
Immune checkpoint inhibitors and T-cell-based therapies, both adoptive and engineered, have unlocked new avenues to treat cancer. Due to their novel mechanism of action, these therapies are not associated with the traditional adverse events seen with cytotoxic chemotherapy.10,11 However, inhibition of checkpoint receptors/ligands and introduction of engineered T-cells (e.g., CAR-T) can lead to excessive immune system activation with the upregulation of T-cell proinflammatory responses, cytokine release syndrome, and immune effector cell-associated neurotoxicity syndrome.10,11 This activation of the immune system can result in immune-related adverse events and the precise exact mechanism of these has not been fully elucidated, but it is hypothesized that T-cell activity, autoantibodies, and proinflammatory cytokines likely contribute to their onset. Dermatologic, gastrointestinal, endocrine, and pulmonary-related immune-related adverse events are the most common, but they can manifest in any organ system.
Accurate assessment of immune cell infiltration in the tumor microenvironment, the subtypes of immune cells present, the expression levels of immune escape genes and the overall neoantigen load of the tumor (as estimated by TMB) are emerging as predictive variables that in combination might serve to better inform immunotherapy treatment decisions. It is likely that additional variates will emerge and when combined, enable the development of multidimensional biomarker with significantly enhanced predictive accuracy for treatment type, likelihood of response and potential for adverse events.
Cofactor has built an immune-profiling solution with ImmunoPrism®, that allows many of these immune features to be measured, in parallel, from two sections of FFPE. Importantly, as described above, it’s unlikely that one single measurement provides sufficient context for understanding disease and therapy response, which is what drives us to use machine-learning to build multidimensional biomarkers.
References:
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tumor-infiltrating-lymphocytes
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6527267/
- https://www.ncbi.nlm.nih.gov/pubmed/28152503
- https://www.ncbi.nlm.nih.gov/pubmed/28790445
- https://www.scribd.com/book/392116968/Oncogenomics-From-Basic-Research-to-Precision-Medicine
- https://www.ncbi.nlm.nih.gov/pubmed/25891174
- https://www.ncbi.nlm.nih.gov/pubmed/28463396
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6591437/
- https://www.ncbi.nlm.nih.gov/pubmed/25977340
- https://www.ncbi.nlm.nih.gov/pubmed/30865922
- https://www.ncbi.nlm.nih.gov/pubmed/31187535




