In this series we have provided an overview of some of the key pieces of immune-oncology. It is clear that the field has a long way to go, but has already made a dramatic and promising impact on the field of oncology. The immune system’s role in cancer has been recognized, or at least hypothesized, for over a century. Now, though we have clarity on a cellular and molecular level as to how the immune system interacts with (i.e, can be activated or suppressed by) tumors. With this information, the field has been able to find potential openings for stimulating immune responses to a few types of cancers (non-small cell lung cancer and melanoma in particular). Today, we have several approved therapeutics designed to target immune checkpoint blockade, such as anti-PD-1 and anti-CTLA-4.
At the same time, more information and insight has led to more questions. The revelation that only a minority (though still a substantial one) of patients respond to any given immune-based therapeutic points to the complexity of the tumor microenvironment and overall heterogeneity within a tumor. Pursuit of additional biomarkers has been the result, with new therapeutics soon to follow. Indeed, numerous companies are developing CAR-T therapies as the field rapidly advances.
Immune-oncology: The Relationship Between Cancer & the Immune System
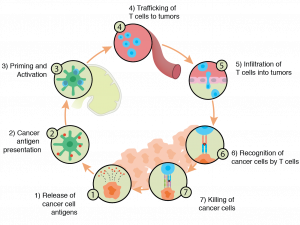
Understanding the relationship between the immune system and cancer has additional implications we have not yet discussed. Cancer is, in many ways, an inflammatory disease. This makes intuitive sense as we see the presence of immune cells. Additionally, as Mantovani et al note, “There are many triggers of chronic inflammation that increase the risk of developing cancer.” Thus, reducing inflammation (in a stronger way than simply taking an OTC medication, of course) through novel therapies holds promise for future interventions in immune-oncology along with things like CAR-T and immune checkpoint inhibitor therapies.
The other issue, often heard when it comes to debates over mammograms, is overdiagnosis. Aggressive screening leads to discovery of tumors. Some of those tumors will turn out to be metastatic, many others won’t. Thyroid cancer is one of these. Diagnosis of the disease has increased dramatically in recent years, leading to “treatment with no effect on outcomes” (Li et al, Radiologic Clinics, 2017). Simply, increased vigilance is leading to discovery of tumors that would not have been dangerous to the patient. Granted, the issue of overdiagnosis depends on the type of cancer being discussed. For example, a recent review by Monticciolo et al suggested that the overdiagnosis of breast cancer is no more than 10%. The authors considered that a low enough percentage to state that the risk of overdiagnosis does not outweigh the potential benefits of early detection.
On the other hand, too many cancers are discovered after the onset of symptoms, at which point they have reached a later and potentially deadly stage. Therefore, it is critical to not just discover the presence of cancer, but to determine whether it will be a problem and what to do about it. As we have seen, immune profiling holds profound promise in the area of immune-oncology.
Immune Profiling
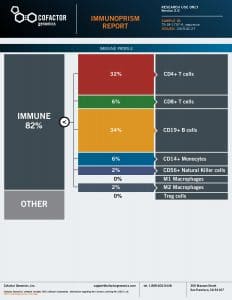
From a methodological standpoint, immune modeling is positioned to serve a central role in both discovery of new biomarkers and quantifying those that are already known. Predictive Immune Modeling provides a level of accuracy, speed and cost-effectiveness necessary to make actionable discoveries and decisions amidst the complexity of tumor heterogeneity. For more information about this method, watch the recorded talk at IO360, here.
Specifically, RNA-seq allows for both diagnosis/prognosis, and discovery. By combining RNA-seq data with other inputs using machine learning, we can 1) understand a patient’s immune profile prior to treatment, and therefore potentially inform that treatment; and 2) identify new prognostic and potentially diagnostic biomarkers. We recognize that this will be accomplished by combining robust molecular protocols with advanced analytics, to deliver this information in an easy-to-interpret way.
As discussed throughout this series, our ImmunoPrism™ assay is designed to profile the immune system in a better way and provide more predictive, multidimensional biomarkers. ImmunoPrism can be used on fresh-frozen or formalin-fixed paraffin-embedded samples, which have in the past been challenging to work with in terms of both RNA extraction and analysis – and impossible to analyze via flow cytometry. Furthermore, very little sample is needed – ImmunoPrism only requires nanograms of RNA. And from that minuscule sample, we can provide immune profiling and escape gene information. Importantly, ImmunoPrism compares favorably with other established methods – ensuring that you can trust the results you collect using this technology. For immune profiling (or what we call “immune cell deconvolution”), our technology offers results that are highly correlated to flow cytometry, as well as other RNA benchmark tests. Importantly, we’re using some of the most sophisticated molecular and informatics technologies – including machine learning – which enable us to provide more robust, specific, and nuanced results, as compared to other technologies.
What this all means is that researchers and clinicians now have access to the multidimensional analysis of tumors we described in the Mutational Burden section of this series, but these multiple analyses only require one sample. Furthermore, the thousands of clinical archives and patient samples stored as FFPE may now be comprehensively profiled – with high precision immune composition analyzed. We are excited about the possibilities this presents to both the translational and clinical communities, and look forward to working with you to drive the field of immune-oncology forward.
Please contact us ([email protected]) with any questions about ImmunoPrism and how it might apply to your work.

