 The human immune system depends on one basic requirement to function. It’s described in every school textbook, but the simplicity of the idea can still get lost because the reality of immunity is so complex. In short, everything comes down to the body’s ability to distinguish “self from non-self.” It’s a relatively easy concept to think about when dealing with truly “non-self” agents such as bacteria and viruses. When it comes to immune-oncology, though, the whole idea becomes quite strange.
The human immune system depends on one basic requirement to function. It’s described in every school textbook, but the simplicity of the idea can still get lost because the reality of immunity is so complex. In short, everything comes down to the body’s ability to distinguish “self from non-self.” It’s a relatively easy concept to think about when dealing with truly “non-self” agents such as bacteria and viruses. When it comes to immune-oncology, though, the whole idea becomes quite strange.
Put aside the years of scientific training and consider just the simple version of the story: a human cell is damaged, whether due to environmental causes or inherited factors. Perhaps that one piece of damage doesn’t do much, but over time the problems accumulate. Eventually, enough damage occurs, enough changes arise, that the cell isn’t fully “self” any longer. At the same time, it retains much of its original appearance, allowing it to potentially evade detection and immune attack. Additionally, its base function remains intact but altered, creating a significant threat to the organism by hijacking natural processes (angiogenesis, for example). Like watching the genesis of a comic book villain, the cancer cell retains enough of its former self to know how to manipulate its environment, and is alien enough to do so for nefarious purposes.
Avoidance and Suppression
That manipulation can take one of two general forms: either avoidance or suppression of an immune response. Tumor cells may express low levels of chemokines, which would normally recruit CD8+ T cells to the site of the tumor. Without the presence of those chemokines, no inflammatory response can occur, leaving the tumor more or less free from harassment by T cells. Other tumors avoid destruction by expressing proteins that inhibit responses from immune cells. Immune cells, particularly T cells, are present at the site of a tumor, but they are held at bay.
Of course, while cancer represents an immune system failure, not every transformed cell is able to establish a tumor. Two overarching reasons for this: 1) the cancer cells are found and destroyed by immune cells; 2) the environment where they land is hostile, or at least not advantageous, for establishing a tumor.
In the first instance, natural killer (NK) cells provide the front line of defense against cancerous cells. From there, the adaptive immune system kicks in when tumor antigens are presented to cytotoxic T cells. These cells then recognize and – hopefully – attack tumor cells.
What all this means is that the immune system has the necessary capability to mitigate tumorigenesis and/or attack tumors when they are established. However, there are times when – in keeping with the comic book analogy – a sidekick is needed to boost the hero’s effectiveness. On the other hand, not all immune infiltrate is beneficial. In yet another layer of complexity, the “immune contexture” (Galon et al and Fridman et al) can include components both helpful and deleterious to the host.
This is the purpose of immune-oncology. First, it is important to study the interplay between immune system and cancer. Understanding how a tumor avoids or impedes immune detection, as well as how it interacts with its surrounding environment to thrive is vital. We can use this understanding to create tools to boost the immune system’s native capacity to attack cancer cells.
Additionally, immune-oncology can provide clinicians with far more granular prognostic capabilities. Medicine needs immune-oncology because, while valuable, traditional imaging and pathology do not provide a complete picture of a tumor. Broadly speaking, traditional detection methods are diagnostic and somewhat prognostic. However, genetic and immune profiling is necessary to get a lock on both the specific diagnosis and the prognosis. And, as the field advances, to provide prescriptive power. To know precisely what intervention is needed, clinicians must know precisely what type – and subtype – of disease they are looking at.
The Tumor Microenvironment
With regards to the area surrounding a tumor, we cannot simply tally up the number and kind of mutations in a tumor cell and claim to have an understanding of how the tumor works. That approach is necessary but not sufficient. On top of profiling the genetics of a cancerous cells, we must also investigate how that cell interacts with those around it. In particular, how it interacts with stroma and non-tumor cells – collectively, the tumor microenvironment.
Expression levels of immune-biomarkers are important to understanding how well a particular microenvironment may be to support a tumor. Part of the success of a cancer cell developing into a primary tumor or landing in a potential metastatic site is determined by the stroma. Simply, is the makeup of stromal cells hospitable enough to support a tumor? A significant determinant of success, though, depends on the other part of the microenvironment – the immune cells present there. Jarret Glasscock, CEO of Cofactor, dives into this topic in a recorded talk at IO360 (watch the full video here.)
We will explore the details of how this all works in more depth throughout the following parts of this series. However, we will close this introduction with a few key highlights that provide a bit more insight into the field of immune-oncology.
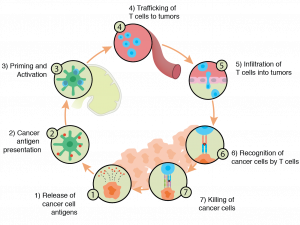
Cancer Immune Cycle
First, it is important to understand the tumor-related signals the immune system responds to. In their excellent review, Lu et al notes that two primary categories of markers exist. TumoqAr-associated antigens are “expressed in some normal tissues at low levels but are over-expressed in malignant cells.” On the other hand, neoantigens are novel proteins, exclusive to a cancer cell and not found on healthy cells. Neoantigens arise out of mutations to a particular gene that lead to alterations in the amino acid sequence. From an immune perspective, these represent better targets because there can be no tolerance due to naturally occurring levels of a protein as seen in antigens. Put another way, neoantigens may be recognized as “non-self.”
The research community is therefore shifting its focus from tumor antigens to neoantigens. Because the former are “self” the immune system has, or can build, tolerance to them. In addition, an unsigned Nature Biotechnology editorial, points out that neoantigens “also produce fewer toxicities arising from autoimmune reactions to non-malignant cells.”
Adoptive Cell Transfer
To push the immune system towards a more effective response against tumor cells through recognition of neoantigens, researchers have developed various forms of adoptive cell transfer (ACT). The most successful to date is CAR-T therapy. (For more information on how ImmunoPrism can be used in CAR-T therapy, see this blog post). T cells isolated from a patient are modified to express chimeric antigen receptors (CAR), synthetic molecules designed to bind a specific tumor-associated antigen or neoantigen. Once the CAR is stably expressed, the T cell population is expanded and then transferred back into the patient. Now, the individual is carrying an arsenal of immune cells built specifically to target her tumor.
Another, older version of ACT involves isolating naturally occurring tumor-infiltrating lymphocytes (TILs), often comprised of CD4+ and CD8+ T cells. As Rosenberg and Restifo note, melanoma has been by far the most successful application of TIL therapy. Today, researchers are refining these basic methods to increase efficacy and diversify the range of cancers that can be treated via ACT. Another version of this (Lu et al) that has been recently published involves boosting the effectiveness of TILs by removing them from the patient, modifying them to express relevant T-cell receptors (TCRs) that recognize neoantigens, and then transferring them back to the patient. This TCR therapy differs from CAR-T in that the antigens expressed are natural rather than synthetic. With these technologies coming on line, a growing list of cancers are now possible targets for ACT and, specifically, CAR-T.
Additional Interventions in Immune-oncology
The last thing we will mention in this intro is the use of drugs to alter immune responses to cancers. In addition to using live T cells as the therapeutic intervention, there are antibodies that target immune checkpoint proteins to enhance the patient’s immune response.
PD-1 and CTLA-4 are by far the most well-established immune checkpoint inhibitors to date. Immune checkpoints function under normal circumstances to prevent over-activation of an immune response. However, as Mahoney et al note, they “also appear to be a means by which tumors evade the immune system.” Anti-PD-1 and -CTLA-4 are used to block the immune checkpoint and thereby allow T cells to move forward in their response to a tumor. As above, PD-1 antibodies have been most effective in melanoma, although several other cancers such as colorectal cancer, renal cell carcinoma, and some forms of prostate cancer and lung cancer have been investigated (Brahmer et al).
As the field of immune-oncology advances, the goal is to take these various interventions and do two things: apply them to more and more types of cancer and expand the repertoire of interventions available as a means to expand the number of patients who can be treated with these very promising technologies. A significant limitation of immune-oncology to date is that the remarkable efficacy of ACT and immune checkpoint inhibitors have so far been limited to a narrow subset of patients (Burugu et al). As the research and clinical communities push forward in their work, new targets – as well as combinations of targets – are being discovered that will allow for more and more personalized intervention. Ultimately this represents the greatest potential promise of immune-oncology: the chance to realize truly personalized AND precision medicine by tailoring therapies to an individual in order to support her natural immune response to the disease.
A Different Solution

Immune-oncology is a burgeoning area of both research and clinical application. The need to gain deep insight into each patient’s tumor will be critical for diagnostic and prognostic purposes, for determining the appropriate course of therapy, and for developing new interventions. The ImmunoPrism™ assay was built for this purpose and provides actionable, multidimensional biomarkers that can predict responses to drug therapy. Data from each patient cohort is analyzed using our proprietary database of Health Expression Models to provide an individual report summarizing the immune profile of each patient. Comparative studies show that our multidimensional models deliver high sensitivity, specificity, and accuracy over single-analyte approaches. When working with patient cohorts from retrospective studies with clinical outcomes, these individual immune profiles are leveraged using Cofactor’s Predictive Immune Modeling platform. They help identify the most powerful combination of analytes to generate a multidimensional biomarker. The predictive accuracy of this multidimensional biomarker is presented in the Biomarker Report, which includes statistics, selection thresholds, and more.
Importantly, we’re focused on making this all possible with very small amounts of FFPE materials – which has previously been very difficult, if not impossible. We will discuss these in later pieces but if you have questions about ImmunoPrism™ in the meantime, please contact us at [email protected], or fill out a form here.

