Bringing
Confidence
to Cancer
Treatment
Confidence
to Cancer
Treatment
ONCOPRISM
PRECISION THERAPY SELECTION FOR IMMUNOTHERAPY AND TARGETED THERAPIES
Other diagnostic solutions for immunotherapy selection don’t have the resolution you need to make confident treatment decisions. With OncoPrism, you won’t be guessing whether a patient should receive monotherapy, combination therapy, or chemotherapy alone. And, where appropriate, targeted therapies* may be recommended for patients where gene fusions are detected.
OncoPrism is validated for use in recurrent and metastatic head and neck squamous cell carcinoma (RM-HNSCC) and non-small cell lung cancer (NSCLC).
A TRANSFORMATIVE SOLUTION
Validated in a clinical study involving 17 healthcare systems, OncoPrism is:
▪ 4X MORE SENSITIVE THAN TMB
▪ 3X MORE SPECIFIC THAN PD-L1
Multicenter validation of an RNA-based assay to predict anti-PD-1 disease control in patients with recurrent or metastatic head and neck squamous cell carcinoma.
CLEAR, ACTIONABLE RESULTS
OncoPrism delivers clear guidance on immunotherapy selection. OncoPrism predicts a patient’s response to anti-PD-1 immunotherapy and places a patient in 1 of 3 distinct categories.
Immunotherapy response based on PD-L1 CPS score has been explored in various studies, including KEYNOTE-048 and KEYNOTE-689.1,2,3
FUSION DETECTION RESULTS
OncoPrism gives clinicians clarity on treatment decisions and improves their ability to identify responders to monotherapy, helping patients avoid the harmful side effects of chemotherapy.
EXAMPLE REPORT
You will receive a report summarizing the results of the OncoPrism test including
OncoPrism score and immune checkpoint inhibitor response prediction results.
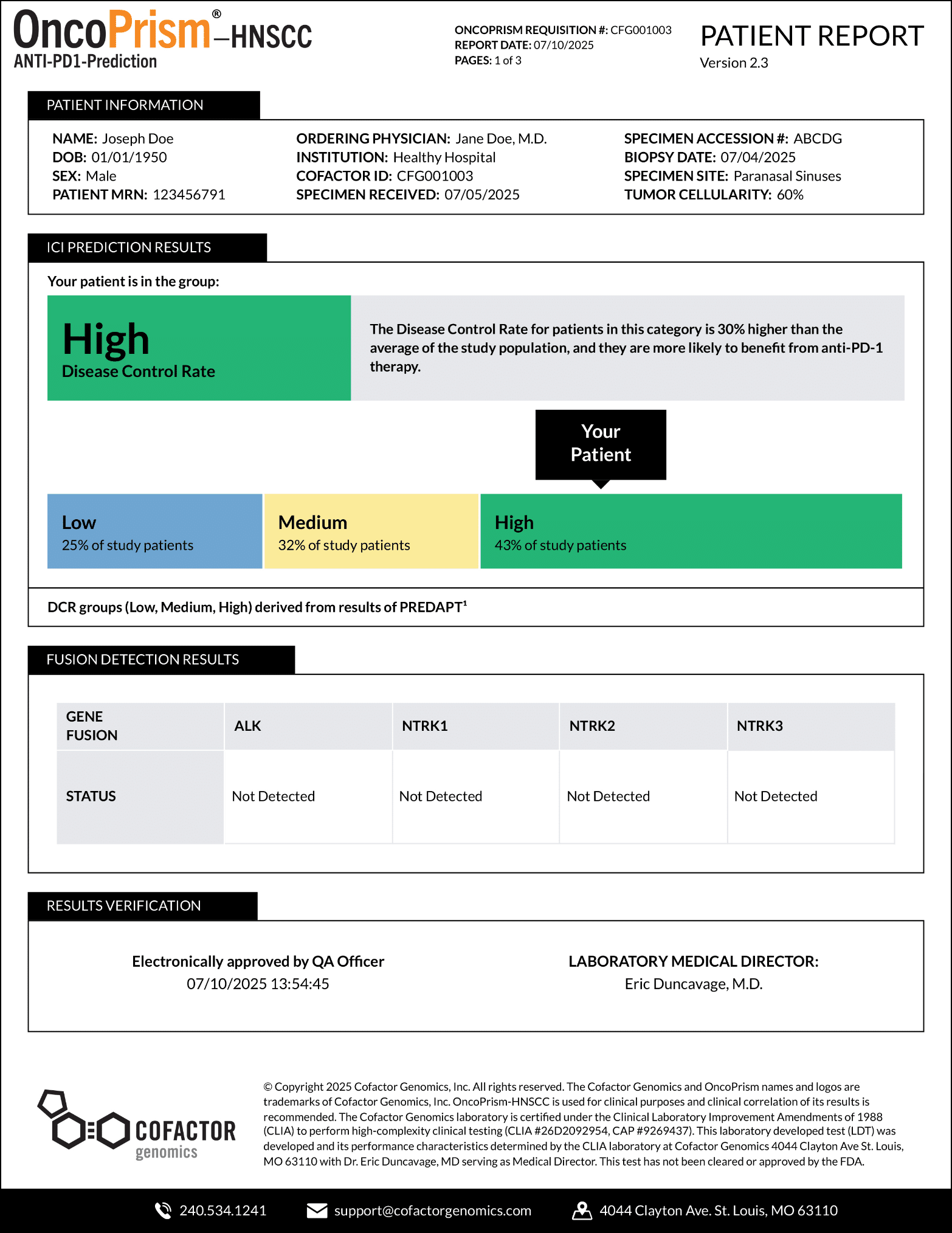
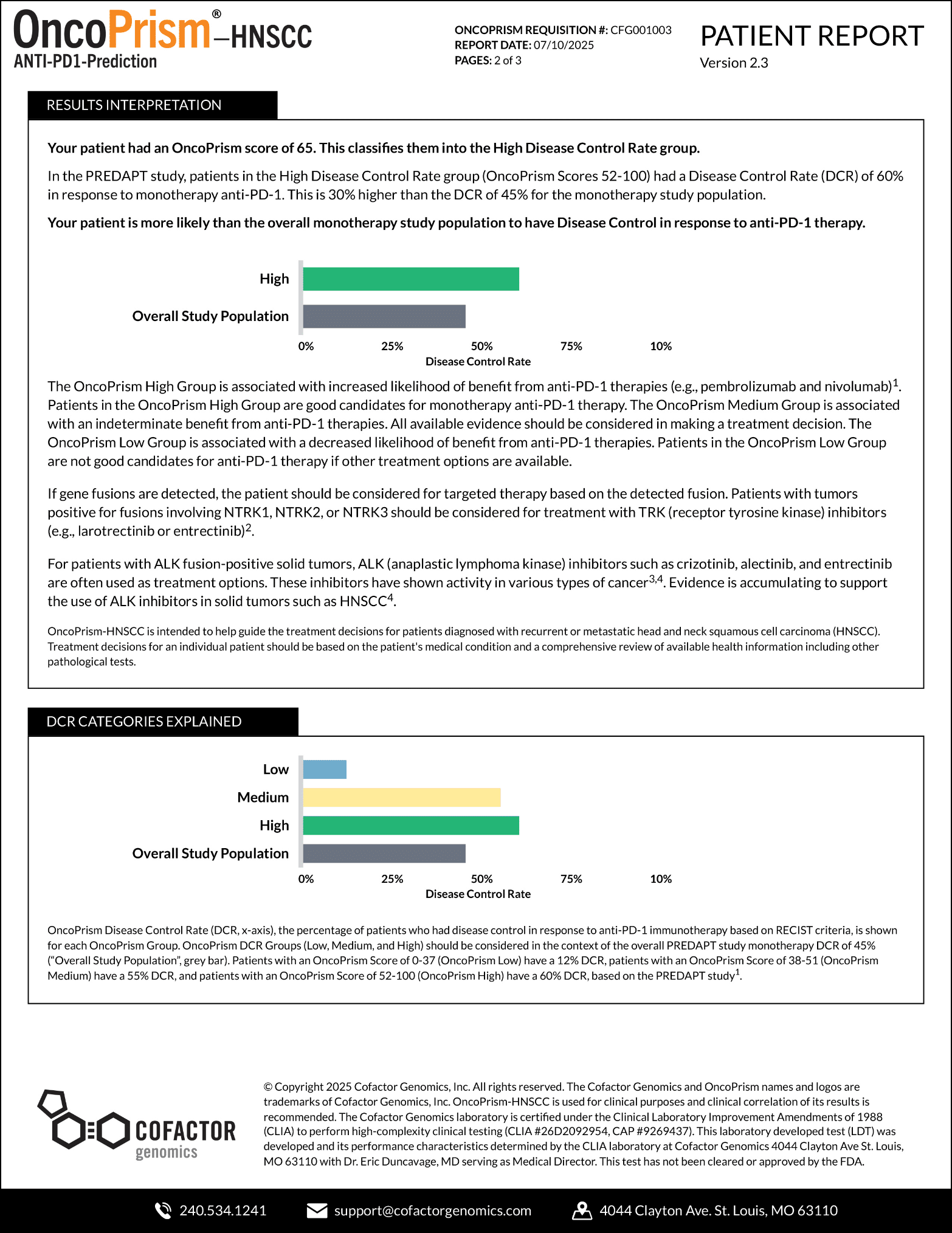


OncoPrism’s Scoring Categories provide guidance on the likely benefit of anti-PD-1 immunecheckpoint inhibitor (ICI) therapies. At the top of the report, your patient’s score will be highlighted.
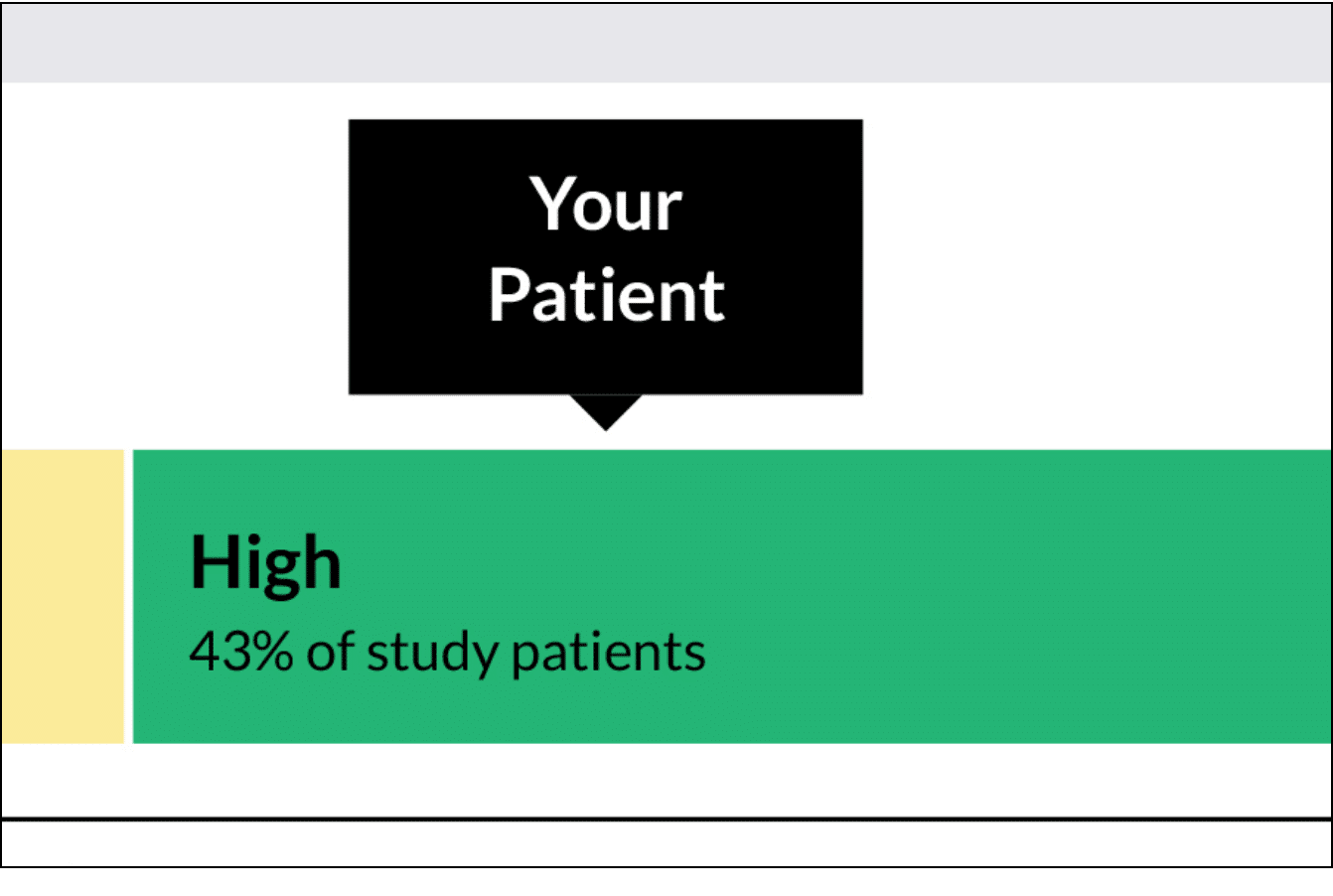
The OncoPrism Scoring Categories are outlined in the report, including the percentage of patients that fall into each category.
- Category 1: Low indicates the patient will be unlikely to benefit from ICI monotherapy.
- Category 2: Medium indicates that the a combination of ICI and chemotherapy should be prioritized. For this category, CPS scores may also be used to provide additional therapeutic guidance.
- Category 3: High indicates that a patient will likely benefit from ICI monotherapy.
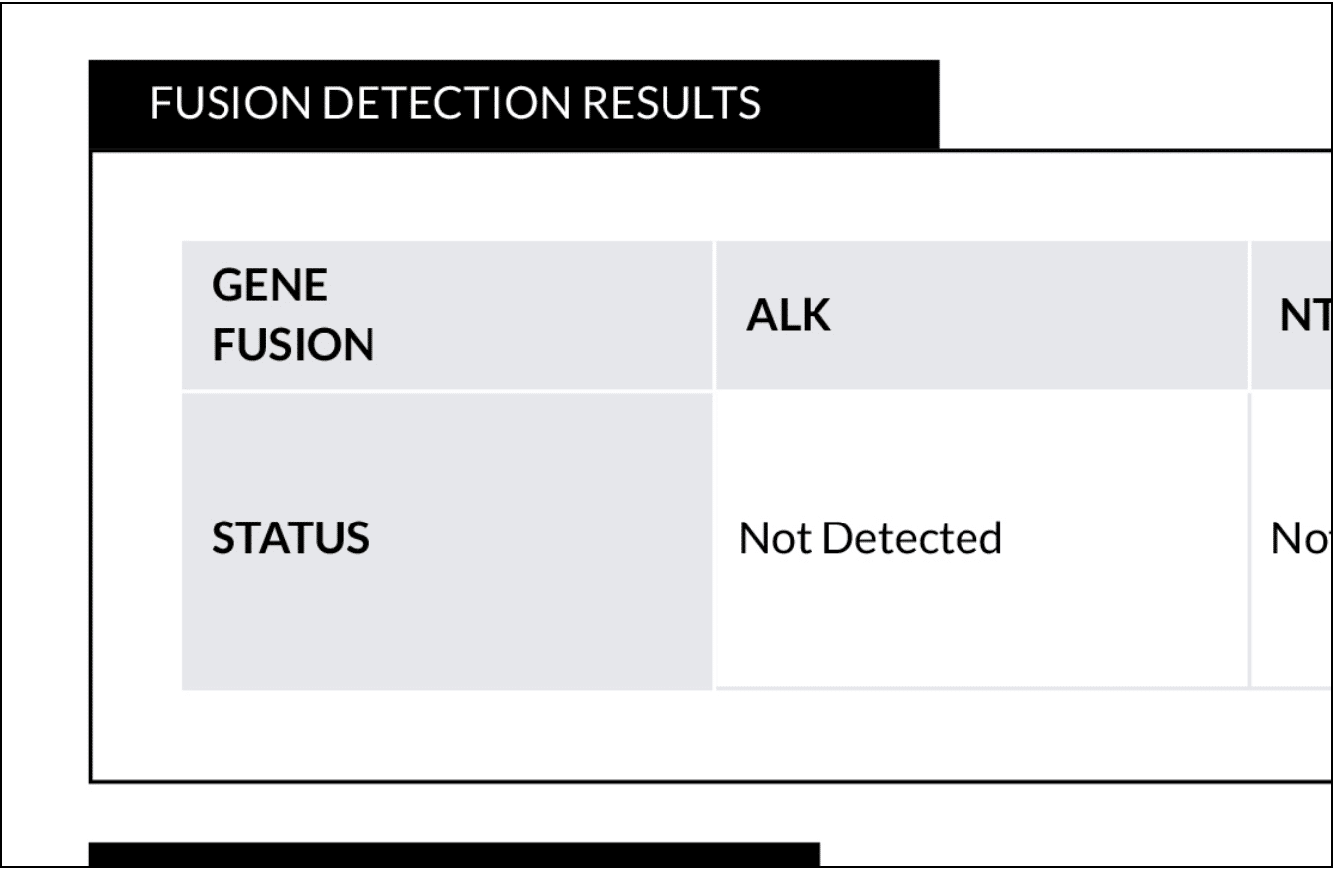
OncoPrism analyzes specific gene fusions expressed and detected in your patient’s RNA. These are reported so that targeted therapies may be prioritized, where appropriate.
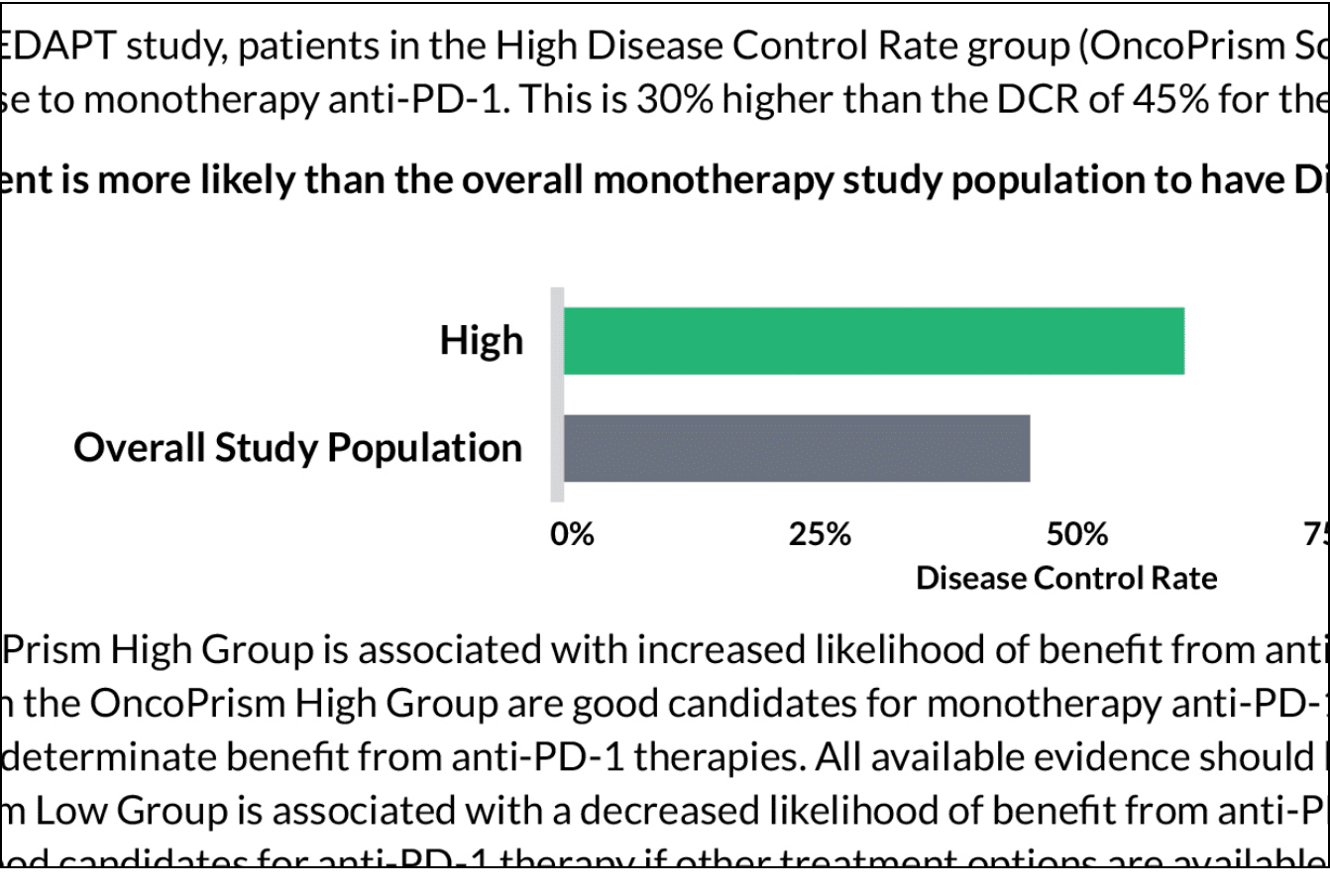
In the results interpretation section of the report, your patient’s OncoPrism score is compared to the overall population studied in the PREDAPT clinical trial. This provides more details on how the therapy response prediction was determined.
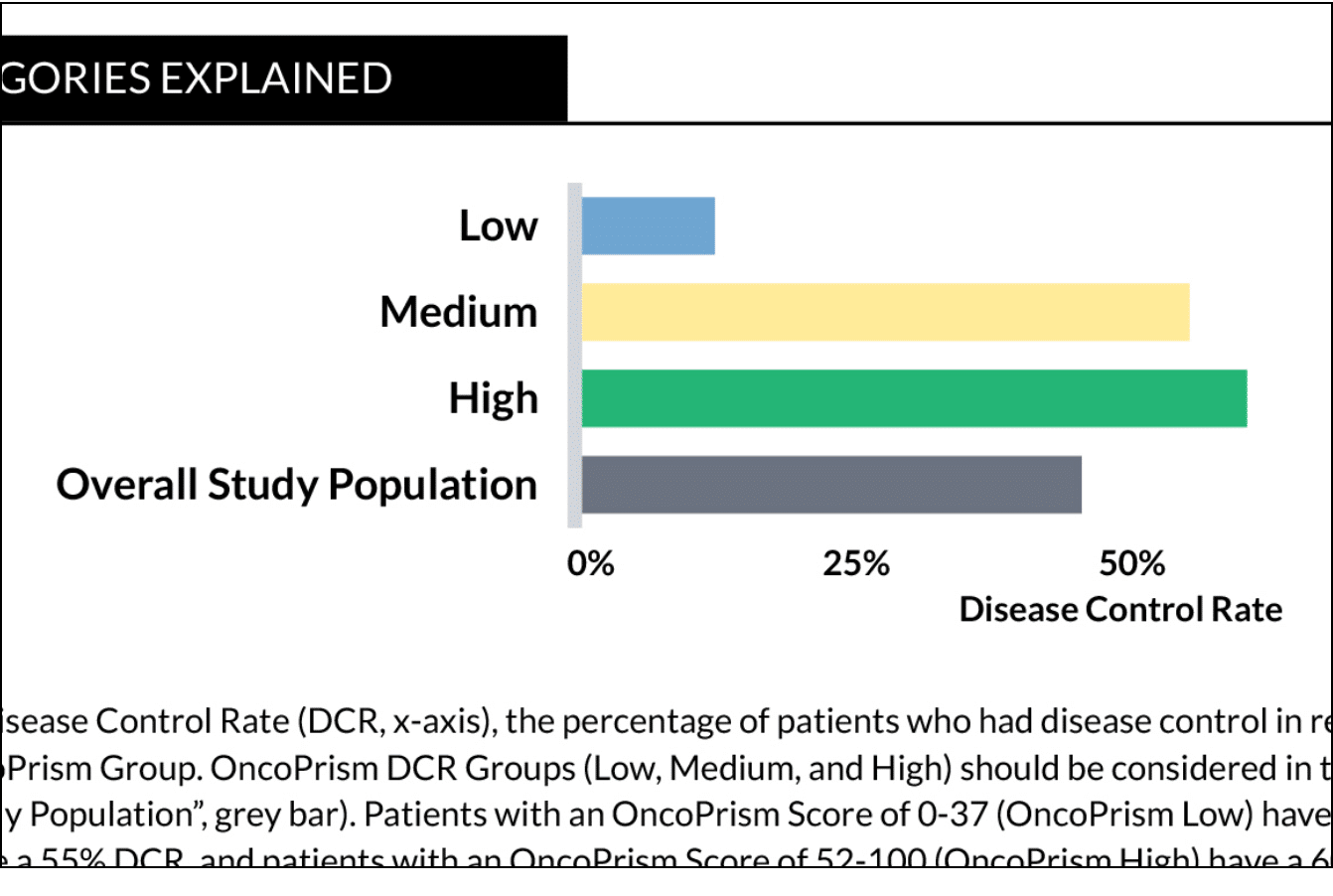
OncoPrism scores were determined using thresholds for disease control rate (DCR) defined based on RECIST criteria measured in the PREDAPT trial.
Additional details on the performance specifications and test methodologies are provided on the final page of the report.
BILLING AND ORDERING DETAILS
OncoPrism has been granted national coverage for Medicare by Palmetto GBA. To order the OncoPrism test, you can order directly from us, register to access the ordering site, or download shipping instructions.
FAQS
DOES THIS TEST REPLACE OTHER CANCER TESTING PROCEDURES?
OncoPrism-HNSCC may be used in conjunction with other tests, and is not designed to replace regular cancer screening tests. The use of the OncoPrism-HNSCC test does not replace, supersede, or otherwise alter the use or frequency of medically established cancer detection modalities. This test is specifically used for selecting a treatment for patients who have already been diagnosed with cancer and are undergoing treatment or follow-up care.
HOW LONG WILL IT TAKE FOR ME TO RECEIVE TEST RESULTS?
Once tumor specimens arrive at the Cofactor Genomics laboratory, they will be processed and analyzed. A report will be sent back to you within approximately 2 weeks.
WHAT SPECIMENS DO YOU ACCEPT? DO I NEED TO PERFORM ANOTHER BIOPSY?
Cofactor Genomics will work with your pathology department to obtain a sample of your patient’s existing tumor specimen.
HOW MUCH WILL IT COST MY PATIENT?
OncoPrism-HNSCC has been granted national coverage for Medicare by Palmetto GBA. Cofactor is committed to working with qualifying patients to manage their out-of-pocket costs. Please contact us to discuss how we can reduce the financial burden for your patients.
WHERE CAN I LEARN MORE ABOUT THE TECHNICAL DETAILS OF THIS TEST?
Information about the technology underpinning the OncoPrism test, along with results from our clinical study may be found in the following peer-reviewed publications:
- Analytical Performance of an Immunoprofiling Assay Based on RNA Models
- Multidimensional biomarker predicts disease control in response to immunotherapy in recurrent or metastatic head and neck squamous-cell carcinoma
- Multicenter validation of an RNA-based assay to predict anti-PD-1 disease control in patients with recurrent or metastatic head and neck squamous cell carcinoma: the PREDAPT study
- Analytical performance of OncoPrism-HNSCC, an RNA-based assay to inform immune checkpoint inhibitor treatment decisions for recurrent/metastatic head and neck squamous cell carcinoma
I’M A CANCER PATIENT, HOW DO I GAIN ACCESS TO THIS TEST?
OncoPrism-HNSCC is available to select healthcare systems through our early access program. Please ask your doctor to order OncoPrism-HNSCC by contacting Client Services.
WHERE CAN I LEARN MORE ABOUT THE PERFORMANCE CHARACTERISTICS OF ONCOPRISM?
Learn more about the performance of OncoPrism-HNSCC by reading our validation papers published in JITC and BMC Cancer.
HOW DOES ONCOPRISM HELP ME DETERMINE THE RIGHT TREATMENT PATH FOR MY PATIENT?
The OncoPrism report and your patient’s score may be used to select the treatment path with the highest likelihood of success.
High Score —> Clinical Benefit from PD-1 inhibitors
Medium Score → Undetermined Clinical Benefit from PD-1 inhibitors; PD-L1 status may be used to direct treatment decision
Low Score → Unlikely Clinical Benefit from PD-1 inhibitors, consider non-ICI treatment options
Positive Result for Fusion Detected → Clinical Benefit from targeted therapies
HOW DO I INCORPORATE MY PATIENT’S PD-L1 STATUS WITH THE ONCOPRISM SCORE?
When a patient’s OncoPrism score is Medium, PD-L1 status measured by CPS may be used to provide additional clarity.
- If a patient with a Medium OncoPrism score has PD-L1 result of CPS≥1, the patient is likely to benefit from PD-1 inhibitors and ICI monotherapy should be prioritized.
- If a patient with a Medium OncoPrism score has a PD-L1 result of CPS < 1, the patient is unlikely to benefit from PD-1 inhibitor monotherapy. Combination therapy (ICI plus chemo), non-ICI treatment options or a clinical trial should be prioritized.
SAFETY/DISCLAIMER
OncoPrism is a next-generation sequencing (NGS) based laboratory developed test (LDT) for head and neck cancer patients with solid tumors. OncoPrism analyzes the RNA from a patient’s solid tumor biopsy to deliver an OncoPrism score which predicts response to immunotherapy response. OncoPrism results are not prescriptive or conclusive for labeled use of any specific therapeutic product. OncoPrism was developed and performance characteristics determined by Cofactor Genomics in their laboratory regulated under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) as qualified to perform high-complexity clinical testing. OncoPrism has not been cleared or approved by the United States Food and Drug Administration (FDA).
*Targeted therapy selection is specific to fusions with approved therapies.
- Burtness B, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 Nov 23;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7.
- Adkins, D. Dr Adkins on the FDA Approval of Pembrolizumab for Neoadjuvant/Adjuvant Treatment in Resectable, Locally Advanced HNSCC. 2025, June 12. https://www.onclive.com/view/dr-adkins-on-the-fda-approval-of-pembrolizumab-for-neoadjuvant-adjuvant-treatment-in-resectable-locally-advanced-hnscc
- Ohara A, et al. Relationship Between Short-Term Outcomes and PD-L1 Expression Based on Combined Positive Score and Tumor Proportion Score in Recurrent or Metastatic Head and Neck Cancers Treated With Anti-PD-1 Antibody Monotherapy. Cancer Rep (Hoboken). 2025 Jan;8(1):e70125. doi: 10.1002/cnr2.70125.